Abstract
Introduction:
Although fludarabine plus melphalan (FM) is one of the most widely used reduced-intensity conditioning regimens for allogeneic hematopoietic stem cell transplantation (allo-SCT), the optimal dose of melphalan for this regimen remains to be elucidated. We therefore compared transplant outcomes of patients with acute myeloid leukemia (AML) receiving reduced-intensity FM based on the total melphalan dose, by using the nationwide registration data of the Japan Society for Hematopoietic Cell Transplantation.
Patients and Methods:
AML patients aged 18 or older who underwent first allo-SCT following reduced-intensity FM from 2003 through 2014 were included. Those who received cord blood as a stem cell source were excluded. A total of 507 patients were identified in the database, and they were divided into lower-dose (80-110 mg/m2, n = 128) and higher-dose groups (120-140 mg/m2, n = 379). Univariate models for overall survival (OS), disease-free survival (DFS), relapse, and transplant-related mortality (TRM) were constructed by including age at allo-SCT (≥60 vs. <60 years), sex, performance status (PS) (2-4 vs. 0-1), hematopoietic cell transplantation-comorbidity index (HCT-CI) (0-2 vs. ≥3), disease status at allo-SCT (standard- [first or second complete remission] vs. high-risk [other]), cytogenetics (intermediate vs. favorable or poor), disease type (de novo vs. secondary), stem cell source (matched related vs. mismatched related, matched unrelated, or mismatched unrelated donor), donor-recipient sex mismatch (female to male vs. other), cytomegarovirus serostatus of a donor-recipient pair, ABO incompatibility, total body irradiation (TBI) (2-4 Gy vs. none), graft-versus-host disease (GVHD) prophylaxis (cyclosporine- vs. tacrolimus-based), period at transplant (2003-2008 vs. 2009-2014), and days from diagnosis to allo-SCT (≥226 vs. <226 days). Factors associated with each endpoint at least borderline significance (p < 0.20) on univariate analysis were subjected to multivariate analysis.
Results:
Patients in the higher-dose group were younger (median age: 58 vs. 61 years, p < 0.001), more likely to have positive-to-positive cytomegarovirus serostatus (55.7% vs. 47.8%, p = 0.045), and less likely to receive TBI (31.7% vs. 82.0%, p < 0.001) than those in the lower-dose group. No significant differences were found in terms of sex, PS, HCT-CI, disease status, cytogenetics, disease type, stem cell source, sex mismatch, ABO incompatibility, GVHD prophylaxis, period at transplant, or days from diagnosis to allo-SCT. The median follow-up period for survivors was 1,030 days (range, 27-4,064).
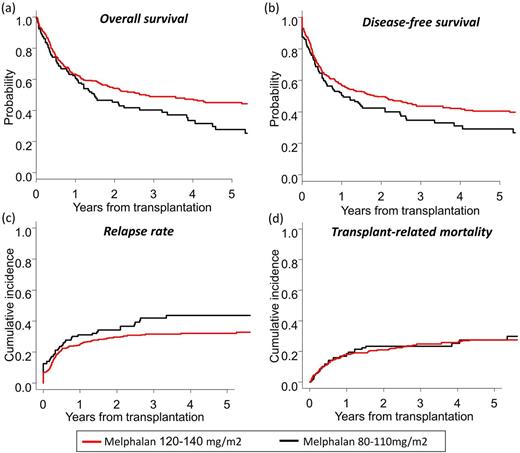
At 3 years, OS and DFS in the higher-dose group were significantly better than those in the lower-dose group (OS: 48.9% vs. 40.3%, p = 0.013; DFS: 43.6% vs. 34.6%, p = 0.035) (Figures a and b). Although there was a trend for lower cumulative incidence of relapse (CIR) for the higher-dose group, TRM did not differ between groups (CIR: 31.5% vs 41.9%, p = 0.085; TRM: 24.8% vs. 23.5%, p = 0.59) (Figures c and d). After adjusting for covariates, higher-dose melphalan was associated with significantly better OS (Hazard ratio [HR], 0.63; 95% confidence interval [95% CI], 0.45-0.87; p = 0.006), better DFS (HR, 0.63; 95% CI, 0.46-0.85; p = 0.003), and lower CIR (HR, 0.54; 95% CI, 0.37-0.81; p= 0.002), while it did not affect TRM (HR, 0.87; 95% CI, 0.56-1.35; p = 0.53). The cumulative incidence of grade II to IV acute GVHD (35.4% vs. 38.3%, p = 0.52) and chronic GVHD (36.0% vs. 34.0%, p = 0.73) were comparable between the higher-dose and lower-dose groups. In subgroup analysis, the beneficial effect of higher-dose melphalan on survival was evident for patients with high-risk disease status (34.4% vs. 17.6% at 3 years).
Conclusion:
For patients undergoing allo-SCT with reduced-intensity FM conditioning, higher-dose melphalan (i.e., 120-140 mg/m2) was associated with lower CIR, better DFS and better OS. Our data support the use of higher-dose melphalan in this setting, especially for patients with high-risk disease status.
Ozawa: Kyowa Hakko Kirin: Honoraria; Janssen Pharmaceutical: Honoraria; Nippon Shinyaku: Honoraria. Atsuta: Otsuka Pharmaceutical Co., Ltd.: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal